History and physics of infrared radiation
From discovery to use as a thermographic camera
Infrared radiation was discovered around 1800, when the Anglo-German astronomer and musician HERSCHEL wanted to measure the temperatures of the different colored parts of sunlight. He let the sunlight fall through a prism and held thermometers in the individual colors of light. He found that the highest temperature could be measured in an invisible region beyond red light. We now refer to this spectral range as the infrared range (infra = beyond) and further divide it into near infrared, mid infrared and far infrared.
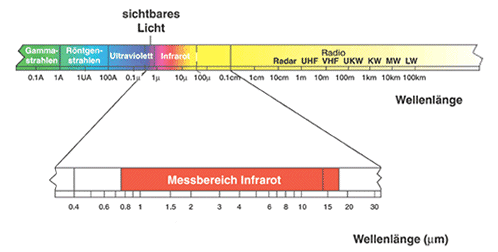
Electromagnetic spectrum: beyond visible red light is the infrared range.
Deeper research into electromagnetic waves, which also includes thermal radiation or infrared radiation, began in the second half of the 19th century. Leading researchers such as KIRCHHOFF, BOLTZMANN, WIEN and PLANCK summarized their discoveries in the laws of radiation.
Towards the mid-20th century, military use of infrared measurement technology began. In the 1960s, the first thermographic devices were developed for non-military use.
A wide range of modern, high-resolution infrared or thermal imaging cameras are now available for different applications. Orglmeister specializes in early fire detection – because early detection of critical temperatures is better than fire detection alone.
The “special” physics of infrared radiation
Imagine standing five meters outside a normal house window. Now you point a thermal imaging camera or thermographic camera in the direction of this window pane and suddenly see yourself. You don’t just see an outline of yourself but also the distribution of temperatures on the surface of your body. Is that what you expected?

Thermography of a house window. The glass pane reflects part of the infrared radiation – the thermographic photographer sees themselves.
Physically, here’s what has happened: The infrared radiation emitted by your body travels a distance of 5 m through the atmosphere to the window pane, is partially reflected there, and then travels another 5 m to the thermographic camera in your hand, where it hits the infrared sensor through the lens.
The internal electronics evaluate the infrared radiation power emitted by your body, calculate the corresponding temperatures and generate a thermal image from this in false-color representation.
Comparing the thermal image with the photo, you will notice that the curtain is not visible on the thermal image. The reason for this is the low thermal transmission (permeability) of glass. The infrared radiation from the curtain doesn’t penetrate the window pane and is therefore not shown in the thermal image.
A thermal imaging camera measures the surface radiation of an object
Thermography can only be used to measure temperatures radiating from surfaces of a material. Remaining hidden from thermography are temperatures of air or gases, temperatures behind obstacles, or on the rear or inside of an infill.
An optimal target for infrared measurement technology is black and has an extremely rough non-reflective surface. Such objects are rare in nature. Objects with these optimal infrared properties are called “blackbody sources”. The name comes from the fact that these sources are so black that the depth of the object is no longer discernible.
For example, if you put a thick layer of plaster on a soccer ball and then drill a 2 cm diameter hole in it, it would be impossible for you to estimate the depth of the hole. Now, if this soccer ball were heated evenly in all places, you would have built an excellent blackbody source that can optimally radiate its infrared energy from this hole. This soccer ball would have an emissivity of ε = 1 and would be called an omnidirectional source.

The PYROcal® test source generates a fixed temperature of exactly 95°C with an emissivity of 0.99. PYROcal® therefore comes very close to being a perfect “blackbody source”. PYROcal® can be used to calibrate thermal imaging cameras.
There are also objects which reflect radiation from other objects, i.e., do not radiate at all. For these so-called “non-black” surfaces, the radiant power must be corrected via the emissivity ε to determine temperatures. Transmission, i.e., the permeability of a material to infrared radiation, also plays a role.
Fortunately, a large number of non-metallic materials have high and relatively constant emissivity in the long-wavelength spectral range, regardless of their surface properties. These include human skin as well as most mineral building materials and paints, rubber, many paints, enamels, etc. Painted, pasted or soiled metallic surfaces are also well-suited for thermographic imaging – here is an example:
A matte black painted body emits more radiant energy than a bright, polished, reflective surface. If you approach a polished stainless steel cube, which is heated to around 100°C, with your hand to a distance of about 10 cm, you will have the feeling that the cube is not particularly hot. If the same cube is now painted with a special matte black paint, the cube feels much hotter at the same temperature and distance from your hand. A thermographic camera would also show these two cubes as having different temperatures.